The menstrual cycle involves complex, dynamic fluctuations in sex hormones, particularly estradiol and progesterone, which exert broad physiological effects beyond reproduction, including significant impacts on glucose levels. People with type 1 diabetes can see changes in their glucose levels during this time—check out professional recommendations of how you can manage it in practice, along with some of the data we know so far.
Recommendations for Healthcare Professionals:
Proactively Address the Topic
Initiate conversations with all women with type 1 diabetes about how the menstrual cycle or hormonal contraceptive use (e.g. challenges during the pill-free interval) may impact their glucose and insulin patterns.
Analyze for specific patterns
Using supportive tools such CGM and insulin dosing/delivery profiles, menstrual cycle phase coding (e.g., early follicular/bleeding, late follicular/pre-ovulation, mid/late luteal phase), tracking apps or diaries, wearable devices (e.g., smartwatch, thermometer), and self-monitored hormone levels can help you compile data and understand patterns.
When interpreting AGP profiles (median and IQR typically over 14 days), adjust the review period to match the individual’s cycle length. Wide IQR bands or an elevated coefficient of variation (CV) may suggest cycle-related glucose variability. Once cycle days are coded, closely review daily CGM data for phase-specific trends.
Promote individualized management and facilitate shared decision-making
Target cycle-phase-specific highs or lows by adjusting insulin timing and dosage. Consider setting phase-specific carbohydrate-to-insulin ratios, insulin sensitivity factors, basal rate profiles, and bolus timing. For users of AID systems, adjust settings as appropriate—such as glucose targets and active insulin time (Medtronic 780G), or basal profiles and correction factors (IQ Control).
Stay informed and recognize knowledge gaps
Acknowledge existing knowledge gaps in this area, stay up to date with emerging research, and take patient-reported challenges seriously. Lack of evidence for average population effects does not mean individual effects are absent – hormonal fluctuations and their potential impact on glucose metabolism vary greatly between individuals and remain incompletely understood.
Document and communicate menstrual cycle-related diabetes management discussions and plans
Record all discussions and management plans related to menstrual cycle effects on diabetes. For women using hormonal contraceptives, clearly document in clinical correspondence when continuous pill regimens or shortened pill-free intervals are implemented as strategies to mitigate withdrawal-related glucose challenges.
While these recommendations can help healthcare professionals work with those in their care to feel more confident while managing menstrual cycle-related changes, we’re still learning exactly how these hormonal changes affect glucose levels. Here’s what we know so far:
Hormonal Dynamics:
Estradiol levels increase steadily during the late follicular phase, peaking sharply just prior to ovulation. Following a brief decline, levels rise again to a secondary, moderate peak during the mid-luteal phase before returning to baseline at menses (up to 10-fold change). Progesterone levels remain low throughout the follicular phase but surge dramatically after ovulation, reaching a mid-luteal peak approximately 20-fold higher than baseline, followed by a rapid premenstrual decline. Follicle-stimulating hormone (FSH) initiates follicular recruitment early in the cycle, while the mid-cycle luteinizing hormone (LH) surge triggers ovulation.
Metabolic Consequences:
These hormonal shifts influence key glucoregulatory processes: insulin sensitivity, energy expenditure, gastric emptying, and appetite regulation. Research on the isolated effects of estradiol – primarily from preclinical studies – indicates that it generally enhances insulin sensitivity, energy expenditure and promotes satiety. However, the combined and dynamic effects of both estradiol and progesterone across the natural menstrual cycle on human glucose homeostasis remain incompletely understood. Additionally, the menstrual cycle influences systemic inflammation, with markers such as C-reactive protein (CRP) rising in the late luteal phase – a factor that may further modulate glucose metabolism. Although the precise mechanisms remain unclear, the cyclical interplay of hormonal fluctuations, inflammatory signals, the autonomic nervous system and their downstream effects on physiology (e.g., insulin sensitivity, energy expenditure) and behaviour (e.g., food intake, physical activity, sleep) collectively contribute to variations in insulin demand.
Challenges in Type 1 Diabetes:
Individuals with a functional pancreas can modulate insulin secretion instantly to meet these changing demands. However, absolute insulin deficiency in type 1 diabetes disrupts this intrinsic adaptation. Evidence from studies, clinical practice, and reports from women with type 1 diabetes consistently indicates that the menstrual cycle challenges diabetes management. Premenstrual hyperglycaemia is frequently reported, and the risk of diabetic ketoacidosis may be elevated during this phase. Data on hypoglycaemia risk are less consistent, with some studies suggesting increased risk during the early follicular phase (post-progesterone decline) or around the pre-ovulatory estradiol peak.
Role of Automated Insulin Delivery (AID):
Recent data illustrate these challenges even with advanced technology (1). Users of the MiniMed 780G AID system required significantly higher total daily insulin doses during the late luteal phase (37.2 ± 11.9 IU) compared to the early follicular phase (33.6 ± 12.2 IU; P < 0.001). Conversely, Time-in-Range (TIR, 70–180 mg/dL) remained similar (83.0% vs. 85.0%; P = 0.101). While these findings suggest that AID systems may partially compensate for the glycaemic effects of menstrual cycle fluctuations, real-world experiences highlight ongoing challenges even with advanced technology (2), prompting important questions: What are the limits of current AID algorithms in compensating for the full magnitude of cyclic insulin requirement fluctuations? How much user intervention (e.g., manual boluses, temporary targets) is still necessary? How do algorithm design and system features influence this adaptability?
Overcoming Knowledge Gaps and Research Challenges:
While methodological challenges in precise menstrual cycle classification, longitudinal hormone monitoring, and comprehensive metabolic data collection have historically limited progress in this field, recent technological innovations now provide transformative opportunities to overcome these barriers. The convergence of high-resolution digital biomarkers (from continuous glucose monitors, AID systems, and smart pens), wearable devices (e.g., smartwatches), advanced nutritional tracking methods, and novel at-home hormone assays enables unprecedented real-world metabolic phenotyping across the menstrual cycle. By integrating these longitudinal multi-modal data streams across multiple cycles (rather than single-cycle observations), researchers can now rigorously characterize menstrual cycle-driven metabolic variability in type 1 diabetes with previously unattainable precision.
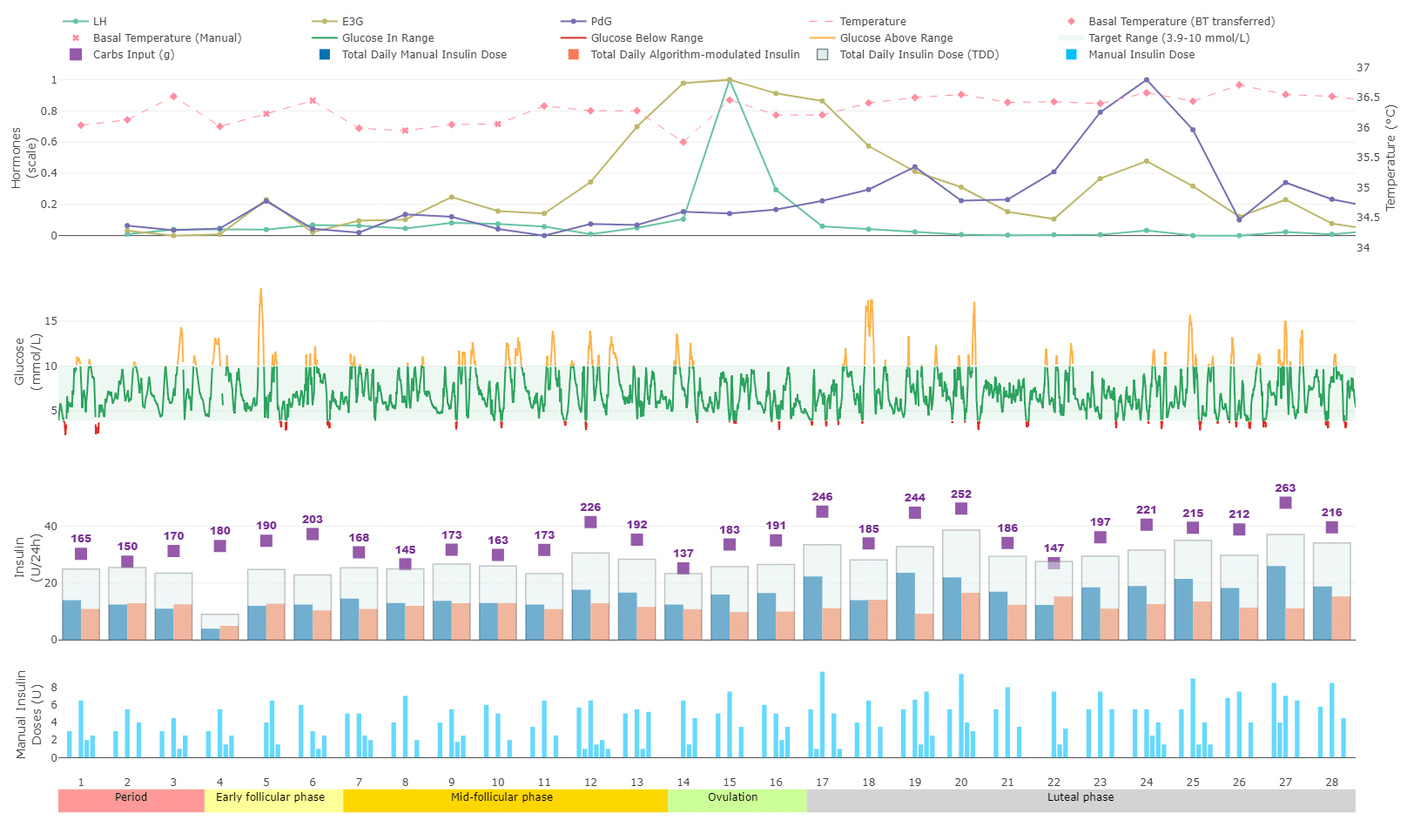
Figure 1 presents an integrated profile of menstrual cycle and therapeutic data across a single menstrual cycle from a reproductive-age woman participating in a clinical study (ID 2023-00430, approved by the Ethics Committee of Bern, Switzerland). The bottom timeline indicates individual cycle days and corresponding menstrual phases. The top panel displays basal body temperature (red line), measured using a Bluetooth-connected thermometer, alongside urinary concentrations of key female sex hormones—estradiol conjugate (light green), luteinizing hormone (LH, dark green), and progesterone conjugate (blue)—self-measured with a point-of-care test system. The second panel shows the continuous glucose monitoring (CGM) trace, with glucose values within the target range (3.9–10.0 mmol/L) highlighted in green. The third panel illustrates daily carbohydrate intake (yellow), insulin administered by the closed-loop system (CamAPS FX, purple), and cumulative manual insulin delivery (grey area). Finally, the fourth panel aggregates manually delivered insulin boluses into 3-hour intervals to illustrate temporal patterns in manual insulin use.
Future impact:
While these insights may provide guidance to better predict and manage menstrual cycle effects, the future lies in empowering women with tools to identify their personal patterns and developing responsive technologies that adapt to individual needs, ultimately optimizing TIR throughout the menstrual cycle.
References
1. Monroy G, Picón-César MJ, García-Alemán J, Tinahones FJ, Martínez-Montoro JI. Glycemic
Control Across the Menstrual Cycle in Women with Type 1 Diabetes Using the MiniMed 780G
Advanced Hybrid Closed-Loop System: The 780MENS Prospective Study. Diabetes Technol Ther.
2025;27(5):395-401.
2. Mewes D, Wäldchen M, Knoll C, Raile K, Braune K. Variability of Glycemic Outcomes and
Insulin Requirements Throughout the Menstrual Cycle: A Qualitative Study on Women With Type 1
Diabetes Using an Open-Source Automated Insulin Delivery System. J Diabetes Sci Technol.
2023;17(5):1304-16.

